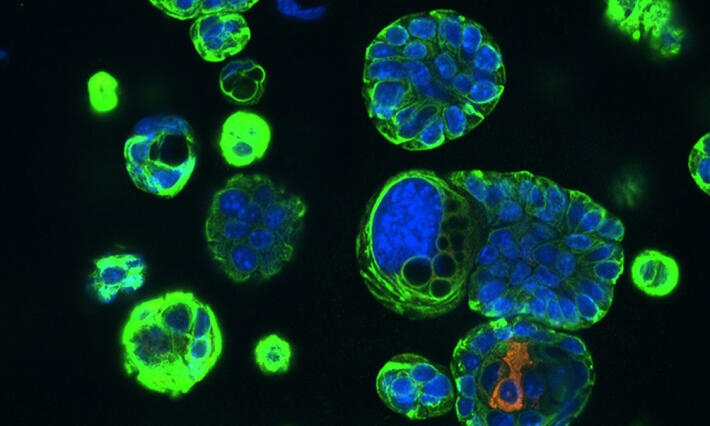
Targeted therapies improve overall outcomes of patients with rare breast cancer subtypes and, in some cases, improve pathologic complete response rates (pCR) and overall survival to rates comparable to those of patients with breast cancer of no special type.
Alexandra Thomas, MD, medical oncologist with the Duke Cancer Institute (DCI) breast oncology program, presented these results from the I-SPY2 trial at ASCO Breakthrough: A Global Summit for Oncology Innovators.
Thomas and her team were able to retrospectively determine the tumor molecular signature and receptor status to classify the response predictive subtype (RPS).